When the speed of car moving down the hill remains constant, its kinetic energy remains same. The gravitational potential energy is converted into internal energy of brake drums. This causes heating of brake drums.


When the speed of car moving down the hill remains constant, its kinetic energy remains same. The gravitational potential energy is converted into internal energy of brake drums. This causes heating of brake drums.


A refrigerator is a heat engine which works in opposite to other heat engines like petrol engine and diesel engine. Thus, a refrigerator extracts heat from the freezing chamber, some work is done on it by electric motor and rejects heat into the surrounding air, thus warming the air. Therefore if the door of a running refrigerator is left open, the room will not be cooled down but instead heated slightly.

When the bus is moving, the man is in state of motion along with the bus. The velocity of man´s body is equal to that of the bus. As soon as he gets down and touches the platforms, his feet become stationary while the upper part of body tends to move forward due to inertia of motion and thus he falls forward. Hence, the man getting out of a moving bus has to run in the direction of the bus for some distance.

Initially, the ball is moving with a certain velocity and the player has to apply a retarding force to bring the ball to rest in his hand. As change in momentum dP = F × dt, if he abruptly catches the ball; i.e. dt is small, then he will have to apply a large retarding force due to which he gets hurt. If he lowers his hands, he would have to apply the smaller force for longer time to bring the ball to rest. Hence he would not get hurt.

The detailed step by step description of a chemical reaction is called reaction mechanism.
Substrate + reagent -> intermediate -> product
*Substrate:- The reactant molecule which is attacked by any reagent.
*Reagent:- The attacking species eg. Electrophiles, Nucleophiles, Free Radicals, etc.

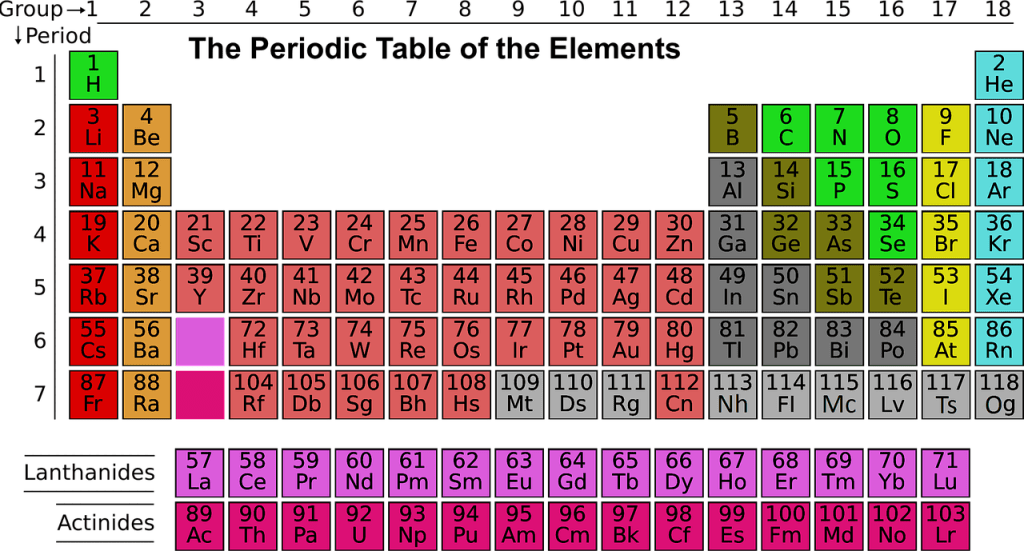
The group of metals in the middle of the periodic table, whose (n-1) d- orbitals are partly filled in atomic state or any ionic state are called transition elements or d-block elements.
Those elements are called transition elements because the general characteristic properties are intermediate between most electro-positive s-block elements to least electro-positive p-block elements.
There are four transition series:
1.First Transition Series(3d-elements): It contains ten elements from Scandium(At.no.21) to Zinc(At.no.30).
*Where At.no. means Atomic Number
2. Second Transition Series(4d-elements): It contains ten elements from Yttrium(At.no.39) to Cadmium(At.no.48).
3.Third Transition Series(5d- elements): It contains 10 elements from Lanthanum(At.no.57) and Hafnium(At.no.72) to Mercury(At.no.80).
4.Fourth Transition Series(f-block elements): It contains Lanthanides and Actinides.
Note:-Non-typical or pseudo transition elements are Zinc(Zn), Cadmium(Cd) and Mercury(Hg).