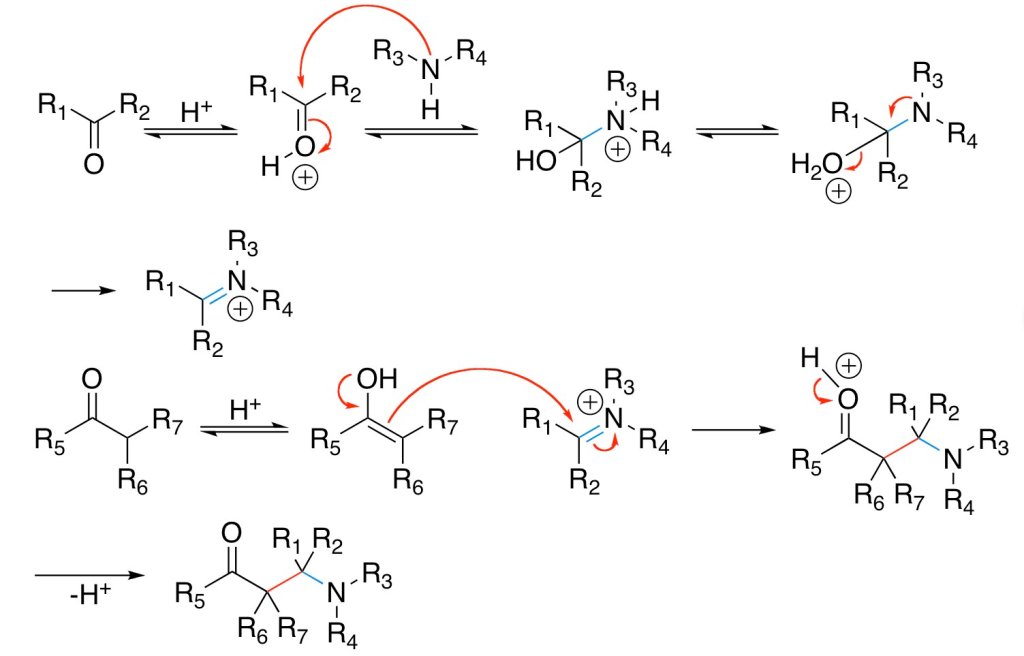
Initially, the ball is moving with a certain velocity and the player has to apply a retarding force to bring the ball to rest in his hand. As change in momentum dP = F × dt, if he abruptly catches the ball; i.e. dt is small, then he will have to apply a large retarding force due to which he gets hurt. If he lowers his hands, he would have to apply the smaller force for longer time to bring the ball to rest. Hence he would not get hurt.