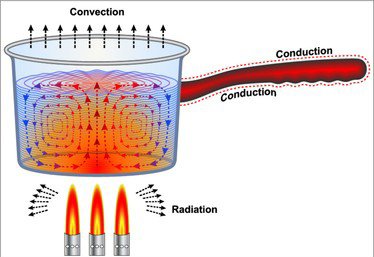
When ice melts, its temperature does not rise and hence its kinetic energy remains constant. The amount of energy taken by the ice is used in deriving the molecules of ice apart and it is stored in the form of potential energy. Thus, heat can be said as a form of potential energy.