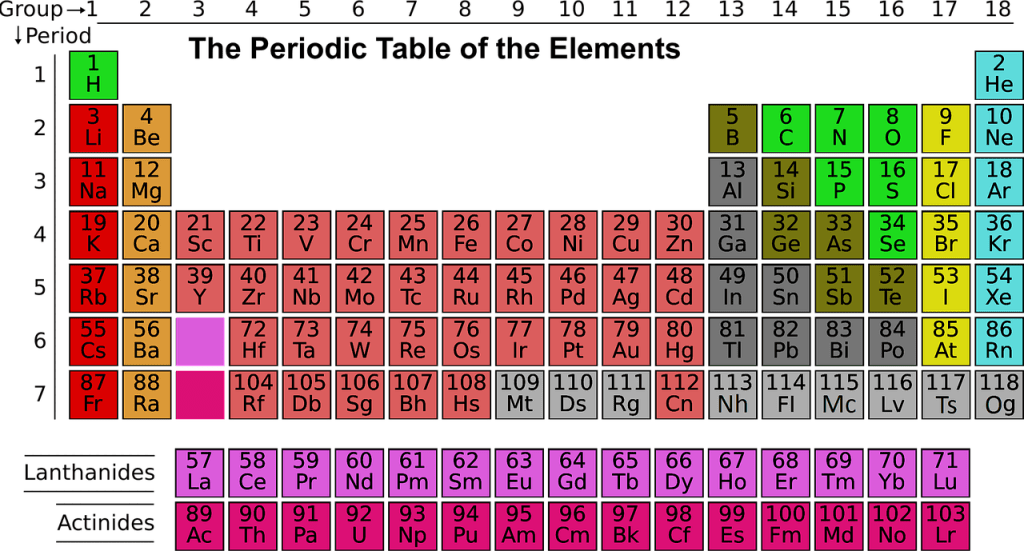
The group of metals in the middle of the periodic table, whose (n-1) d- orbitals are partly filled in atomic state or any ionic state are called transition elements or d-block elements.
Those elements are called transition elements because the general characteristic properties are intermediate between most electro-positive s-block elements to least electro-positive p-block elements.
There are four transition series:
1.First Transition Series(3d-elements): It contains ten elements from Scandium(At.no.21) to Zinc(At.no.30).
*Where At.no. means Atomic Number
2. Second Transition Series(4d-elements): It contains ten elements from Yttrium(At.no.39) to Cadmium(At.no.48).
3.Third Transition Series(5d- elements): It contains 10 elements from Lanthanum(At.no.57) and Hafnium(At.no.72) to Mercury(At.no.80).
4.Fourth Transition Series(f-block elements): It contains Lanthanides and Actinides.
Note:-Non-typical or pseudo transition elements are Zinc(Zn), Cadmium(Cd) and Mercury(Hg).